Industry’s User Fees Fail to Improve FDA’s Approvals of Medical Devices
In June 2012, I wrote an analysis of the effect of user fees, paid by the medical-device industry, on the Food and Drug Administration’s behavior with respect to approving new medical devices. My conclusion: The FDA had sucked up the dollars without increasing its productivity.
New research, commissioned by the California Healthcare Institute, a trade association, confirms that the industry’s user fees are disappearing into a black hole. Despite putting a positive spin on the behavior of the regulator, which has a choke-hold on the industry’s ability to launch new products, the evidence indicates that the millions of dollars that the industry has paid to the FDA have not improved its performance:
- Data suggests that the PMA classes of 2011 and 2012 will show the best overall review-time performance of the device user fee era. 2013 shows even further improvement, however, it is premature to determine given the number of products still awaiting a decision.
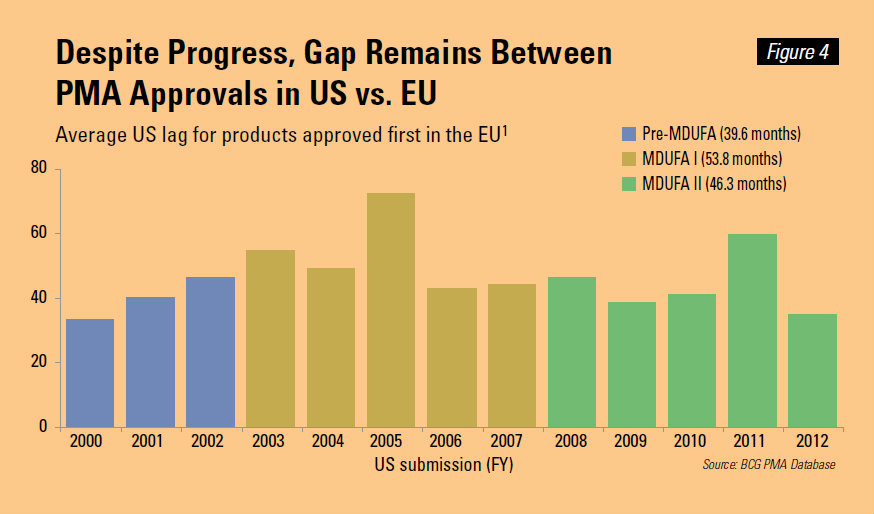
- When comparing PMA submission trends at the FDA with those in Europe, we see that the 3-5 year lag between approvals in Europe and subsequent US approvals hasn’t improved during the user fee era, which began in 2002.
- After holding steady between 2000 and 2006, 510(k) clearance times lengthened dramatically, with review times in 2010 being 60 percent longer than in 2000. Even with some improvements over the past couple years, 510(k) review times continue to remain far higher, and processes are still viewed as less predictable, than during the pre-device user fee era.
- After climbing steadily since 2005 and peaking in 2010, the Agency’s 510(k) backlog has begun to improve ― especially for those pending for more than 90 days. However, by nearly any measure, there is still much work to be done before the 510(k) process is considered back on track.
- Review divisions have varied markedly in meeting their PMA MDUFA performance goals. Similar variances exist across branches for 510(k) decisions. Encouragingly, the most recent years’ data shows a trend towards narrowing those gaps.
Of course, it’s good to see optimism expressed by the industry. However, it is becoming very clear that user fees have not changed the incentives of the FDA. One major reason that the industry lobbied to pay user fees in 2002 was that medical devices in Europe were being improved faster than in the U.S. Figure 4 makes it clear that there has been no real improvement in this gap since 2002.


Where are the user fees supposed to go?
They go to pay the FDA to do its job: Review applications for new medical devices. Advocates argue that appropriations are not adequate to fund the FDA’s operations.