Congress Should Take Steps to Make Drugs More Affordable
 As Republicans squabble about how to repeal & replace Obamacare, lost in the debate is the way most Americans actually access our health care system. In any given year, most people don’t ride in the back of an ambulance heading to the ER. Nor do they convalesce in a hospital bed. Most of the medical care Americans receive is not even provided in doctors’ offices. The most common way Americans access our health care system is by taking a pill.
As Republicans squabble about how to repeal & replace Obamacare, lost in the debate is the way most Americans actually access our health care system. In any given year, most people don’t ride in the back of an ambulance heading to the ER. Nor do they convalesce in a hospital bed. Most of the medical care Americans receive is not even provided in doctors’ offices. The most common way Americans access our health care system is by taking a pill.
Drug therapy is convenient and usually relatively cheap. Most of the drugs Americans take do not even require a prescription — but a few come with price tags that make your mortgage look cheap. Patients increasingly experience sticker shock at the pharmacy counter because rising health plan deductibles have forced them to pay more of their drug costs out-of-pocket. This isn’t necessarily bad; cost-sharing makes it harder for drug makers to jack up prices without anyone noticing.
A significant driver of high drug prices is the excessive regulatory regime at the U.S. Food and Drug Administration (FDA). Few people notice when the FDA drags its feet and drugs take a dozen years to reach the market; yet, the agency suffers a severe public relations backlash when an approved drug causes unforeseen side effects. Thus, the FDA has an incentive to error on the side of caution. A drug that makes it through the FDA approval process is guaranteed years of high monopoly prices due to regulatory barriers that limit competition. To boost competition, a streamlined path to faster drug approval is badly needed. In a market with numerous firms competing to sell drugs, high prices should entice more firms to enter the market. Guidance from Congress would reduce this regulatory bottleneck and refocus the agency’s priorities.
One place priorities need to be realigned is so-called “me-too” drugs, developed to compete with existing drugs. A little over a decade ago, some people from the public health community (with a poor grasp of economics) began to criticize drug makers for developing drugs similar to those already on the market. These busybodies never understood how competition benefits consumers rather than wastes resources. They claimed me-too drugs offer little added benefit and the funds would be better spent researching novel drug therapies. That’s like saying Ford and Chevy should not build SUVs because Jeep was already building an SUV prior to the Explorer and the Tahoe.
The reality is that the first-in-class drug is often inferior to similar but competing drugs that came later. The FDA’s bias toward approving newer, first-in-class therapies has increased approvals for expensive drugs to treat rare diseases, which are at a historic high. Approvals of me-too drugs are down, however, which limits competition within drug classes, leading to higher prices, and limits patient choices.
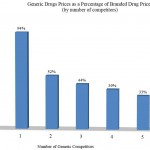
In response to increased public scrutiny about high drug prices, drug makers began looking for a scapegoat and soon blamed “the middleman.” The middleman is an old boogeyman most consumers have heard of. However, the claim is mostly bogus. What drug makers call the middleman is the industry’s supply chain. The drug supply chain starts with the makers of raw ingredients and includes drug makers themselves, drug wholesalers, pharmacies and drug benefit plans sponsored by employers and insurers. Common sense suggests that the purveyors of raw ingredients, drug makers, wholesalers and pharmacies all want to maximize their profit by charging prices as high as their customers will pay. However, in a competitive market with numerous firms competing to sell drugs (and raw ingredients), high prices entice other firms to enter the market. Consider this: when a branded drug faces competition from only one generic, the price of the competing generic is 94 percent of the price of the brand-name drug. By the time there are two generic competitors, the price is one-third. Eliminating the FDA bias against me-too drugs could slow price increases long before drugs face generic competition.
Furthermore, increasing the number of drugs within a drug class is also beneficial to patients because not all patients respond to a given drug in the same way.
The 21st Century Cures Act, signed into law by President Obama in December 2016, aims to streamline the approval process for new drugs — but will undoubtedly fall short of its goal. An earlier 2012 law pushed the FDA to fast track drugs for “serious or life threatening” conditions, antibiotics and breakthrough therapies. Unfortunately, this process is not available for drugs that are similar to existing drugs and the primary benefit of which may be merely holding older drugs’ prices in check.
A way to rein-in high drug prices is to inject more competition. Newer drugs would face numerous competitors if it didn’t require 12 years and more than $1 billion to bring a drug to market. With increased competition high prices — such as prescriptions that cost $1,000 per pill — would be impossible for drug makers to maintain. Competition works great in that regard.


Bernie Sanders on CNN tonight: “What’s the point of health insurance if you can’t get it when you need it?”
It’s funny how people assume the purpose of health insurance is to pay for every single service a patient may need. Technically, health insurance is supposed to insure you against unforeseen problems and transfer the risk of catastrophic medical problems to a larger group.
Devon – I think a significant part of the conservative drug approval process and culture at the FDA dates back to 1962 when a reviewer named Frances Kelsey stopped Thalidomide from being approved in the U.S. when it turned out to cause horrific birth defects in Europe after being prescribed to pregnant women to combat morning sickness. Nobody at the FDA wants to be the person who signs off on the next Thalidomide. I don’t have a good answer to address this cultural bias sometimes referred to as “Frances Kelsey Syndrome” at the agency.
As for high priced specialty drugs, one way to mitigate aggressive (high) pricing is to just refuse to pay for them if they are deemed too expensive using some rational basis like QALY metrics to make that determination. Specialty drugs account for only 1% of prescriptions written and only 2% of patients need specialty drugs in any given year but they account for one-third of prescription drug costs and that percentage is rising steadily.
One thing you didn’t address is the issue of rebates paid to pharmacy benefit managers and insurers who then pass much or all of the savings on to their self-funded employer clients. Rebates are paid basically as an incentive to drive volume of specific drugs and are usually a percentage of the list price so the rebate recipients actually benefit to some degree every time the list price rises. The problem is that the patient with a high deductible drug plan often gets stuck paying the artificially high full list price. With modern technology, it shouldn’t be all that difficult to redirect the rebate to patients with high deductible plans and adjust their out-of-pocket spending that counts toward meeting their deductible accordingly. Since the patient paid the whole bill up front, the plan won’t be any worse off than if it paid for the drug in full and later received the rebate to offset part of the cost. Epi-Pen is the poster child for this problem.